“We don’t need any energy input, and it bubbles hydrogen like crazy. I’ve never seen anything like it,” said UCSC Professor Scott Oliver, describing a new aluminum-gallium nanoparticle powder that generates H2 when placed in water – even seawater.
Aluminum by itself rapidly oxidizes in water, stripping the O out of H2O and releasing hydrogen as a byproduct. This is a short-lived reaction though, because in most cases the metal quickly attains a microscopically thin coating of aluminum oxide that seals it off and puts an end to the fun.
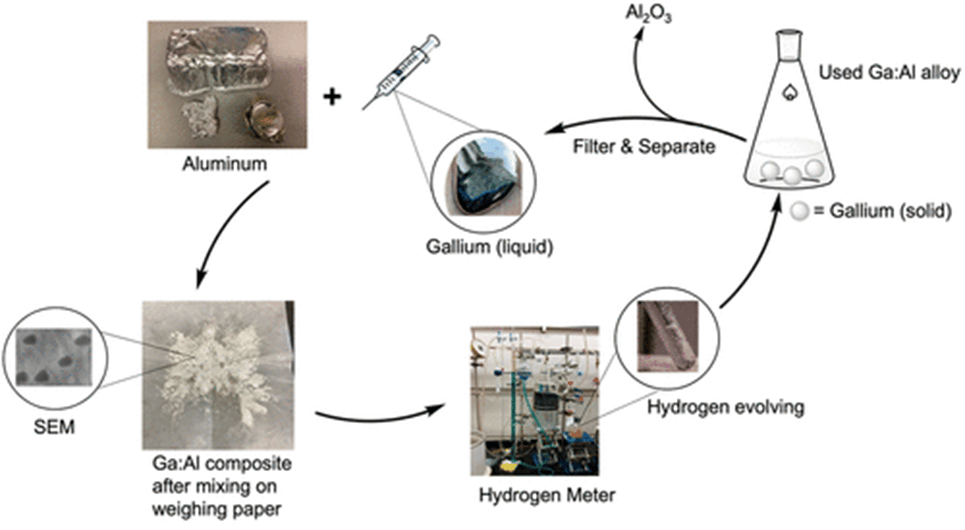
But chemistry researchers at UC Santa Cruz say they’ve found a cost-effective way to keep the ball rolling. Gallium has long been known to remove the aluminum oxide coating and keep the aluminum in contact with water to continue the reaction, but previous research had found that aluminum-heavy combinations had a limited effect.
So when chemistry/biochemistry Professor Bakthan Singaram found out that student Isai Lopez was playing with aluminum/gallium hydrogen production in his kitchen at home, there didn’t seem to be anything particularly special about the idea.
“He wasn’t doing it in a scientific way, so I set him up with a graduate student to do a systematic study,” Singaram said. “I thought it would make a good senior thesis for him to measure the hydrogen output from different ratios of gallium and aluminum.”
When Lopez decided to extend the experiment to test gallium-heavy mixtures, things got a little weird. Hydrogen production went through the roof, and the team started trying to figure out why these mixtures were behaving so fundamentally differently.
After electron microscopy and X-ray diffraction studies, they realized that the most effective mix, three parts gallium to one part aluminum, was indeed doing something the lower ratios weren’t. Not only was the gallium dissolving the aluminum oxide, it was also causing the aluminum to separate into nanoparticles, and keeping them separate.
“The gallium separates the nanoparticles and keeps them from aggregating into larger particles,” Singaram said. “People have struggled to make aluminum nanoparticles, and here we are producing them under normal atmospheric pressure and room temperature conditions.”
With the aluminum so finely separated, its surface area is maximized and the reaction with water was spectacularly efficient, pulling out 90% of the theoretical maximum amount of hydrogen possible for a given amount of aluminum. In a study published in ACS Nano Materials, the researchers report that a single gram of their gallium-aluminum alloy will rapidly liberate 130 ml of hydrogen when placed in water.
University of California Santa Cruz
Remarkably, the water source doesn’t need to be clean, either.
“Any available water source can be used,” reads the study, “including wastewater, commercial beverages, or even ocean water, with no generation of chlorine gas.”
Now, yes, gallium is expensive. But the researchers say it can be fully recovered at the end of the process, and used with fresh aluminum to create more of this remarkable hydrogen-producing alloy. Indeed, the creation of the alloy is extremely easy in and of itself; one simply mixes the gallium together manually with aluminum, including used foil or cans, in the correct ratio.
“Our method uses a small amount of aluminum, which ensures it all dissolves into the majority gallium as discrete nanoparticles,” Oliver said. “This generates a much larger amount of hydrogen, almost complete compared to the theoretical value based on the amount of aluminum. It also makes gallium recovery easier for reuse.”
The team has slapped a patent application on the process, and is beginning to examine how it’ll scale up commercially.
So what are we looking at here? Well, it’s effectively a solid-state way to store and release hydrogen – remarkably, the third hydrogen-storage powder we’ve written about in the last couple of months, or indeed ever. Hydrogen is an important fuel that’ll be necessary in certain applications during the race to decarbonization, but it’s notoriously difficult and expensive to compress into gas, or cryogenically condense into a liquid, for storage and transport.
A hydrogen-storage powder, on the other hand, is much easier and cheaper to handle, potentially changing the cost of working with hydrogen so drastically that new applications become viable. Which is why Deakin’s mechano-chemical ball-milling process, and EAT’s Si+ silicon powder were such a big deal.
And why this UCSC advance could be such a big deal as well. This stuff sounds extremely easy to make, and even easier to use for hydrogen production. It’ll store and travel well for at least three months if stored in cyclohexane gas. The fact that it works in seawater is hugely significant; access to clean water is not the sort of thing you’d want to be staking a volume business on moving forward. The fact that the gallium can be collected and recycled back into the process will help keep costs down. And the fact that the reaction happens at ambient pressures and temperatures means you can get away with less equipment at the pointy end of the whole operation where you actually need the hydrogen.
So how does it measure up against these other two powders? Well, the figures provided allow us at least to take a guess. If you’re treating this stuff as a hydrogen storage medium, then the key metric is probably the mass fraction: for a given mass of powder, how much hydrogen can you get out? Well, if a gram of gallium-aluminum powder produces 130 ml, or 5.4 mmol of hydrogen, that hydrogen would weigh 0.00544 grams.
That’s a mass fraction of 0.544%. Not much chop, really; EAT’s Si+ powder is probably the substance to beat at this stage, at least on this metric, claiming a mass fraction of 13.5%. Of course, there are many other considerations when you’re talking about a commercial energy transport and release cycle – particularly one that’s not fussy about water quality – so there’s definitely still opportunities for this new powder to make a contribution.
The research is published in the journal ACS Nano Materials.
Source: UC Santa Cruz
Source of Article