In what will soon be commonplace in drug research, scientists have used an artificial-intelligence algorithmic program to identify a compound, currently used in antimalarial treatment, that can effectively reverse the bone deterioration of osteoporosis.
The compound, dihydroartemisinin (DHA), is derived from the native Asian plant Artemisia annua L., commonly known as sweet wormwood or sweet sagewort, which has been used in traditional Chinese medicine for more than 2,000 years.
A large collaboration led by scientists at the Peking University School and Hospital for Stomatology and the Peking University International Cancer Center in Beijing have used a deep-learning algorithm they had previously designed, to predict how well different small-molecule drugs could reverse certain gene expression in osteoporosis.
This time, focusing on bone marrow mesenchymal stem cells (MSCs), the team used the AI on profiles of newborn and aged mice and identified DHA as a top-ranked compound.
Bone marrow MSCs are the precursor cells to osteoblasts, which form bone tissue. However, during osteoporosis, this differentiation of the stem cells falters and they turn into fat-producing cells instead of osteoblasts. As such, the wrecking-crew cells, osteoclasts, dominate and bone loss progresses.
“Because BMMSCs provide a continuous supply of osteoblasts for bone repair, it is critical to find ways to restore their functions,” the researchers noted.
In a mouse study, DHA-loaded nanoparticles were injected into animals with induced osteoporosis over six weeks. After this time period, the treatment had significantly reduced expected bone loss and almost completely preserved bone structure.
“To improve the therapeutic efficiency of DHA in osteoporosis, mesoporous silica nanoparticles (MSNs) conjugated with bone-targeting alendronate (ALN) were designed to deliver DHA,” they noted.
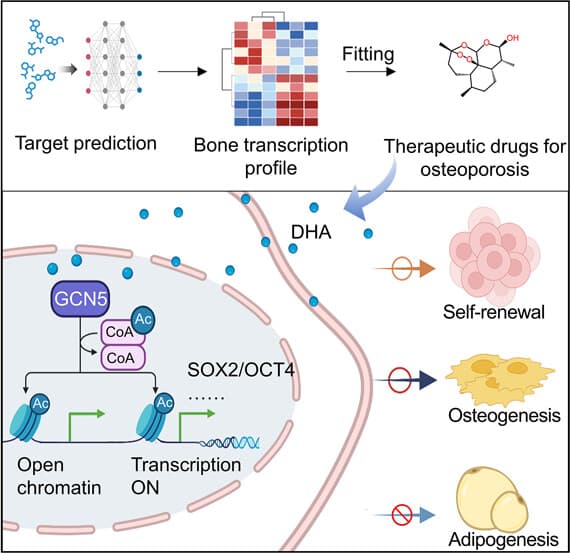
In further tests, the team identified that the DHA interacted with the bone marrow MSCs to maintain ‘stemness’, or ensured they continue to develop into osteoblasts. It also showed no signs of toxicity, setting it up as a very promising safe and effective therapy for osteoporosis.
“Current mainstay drugs for osteoporosis, such as estrogens and bisphosphonates, mainly target hormone deficiency or bone resorption but do not directly restore the stemness and vitality of BMMSCs,” the researchers noted.
Broadly, using AI to pivot existing drugs for new treatments is a quickly growing area of medical research. And leading the way are small-molecule drug discoveries.
Last year a study detailed that biotech companies were using an ‘AI-first’ approach I research, and more than 150 small-molecule drugs were already in discovery stage, with 15 in clinical trials. As well as being hugely cost-effective, AI-assisted drug-discovery research will speed up the process significantly, which is crucial in getting new, life-saving medicines approved for use as quickly as possible.
Some of the discoveries already made by AI that are progressing through clinical trial stages include treatments for obsessive-compulsive disorder (OCD) and idiopathic pulmonary fibrosis (IPF).
Earlier this week, scientists at the University of Cambridge launched Polymatheic AI, a machine learning model to facilitate discoveries across all fields that may be overlooked, by humans specializing in certain disciplines.
The study was published in the journal ACS Central Science.
Source: Peking University School and Hospital for Stomatology via MedicalXpress
Source of Article