Researchers at the University of Southern California looking to crack the renewable energy storage problem have developed a new version of a redox flow battery from inexpensive and readily-available materials.
Though there are huge lithium-ion battery installations from the likes of Tesla that can store energy harvested from renewables like wind and solar, they’re not exactly cheap. The USC researchers looked to an existing design that stores energy in liquid form.
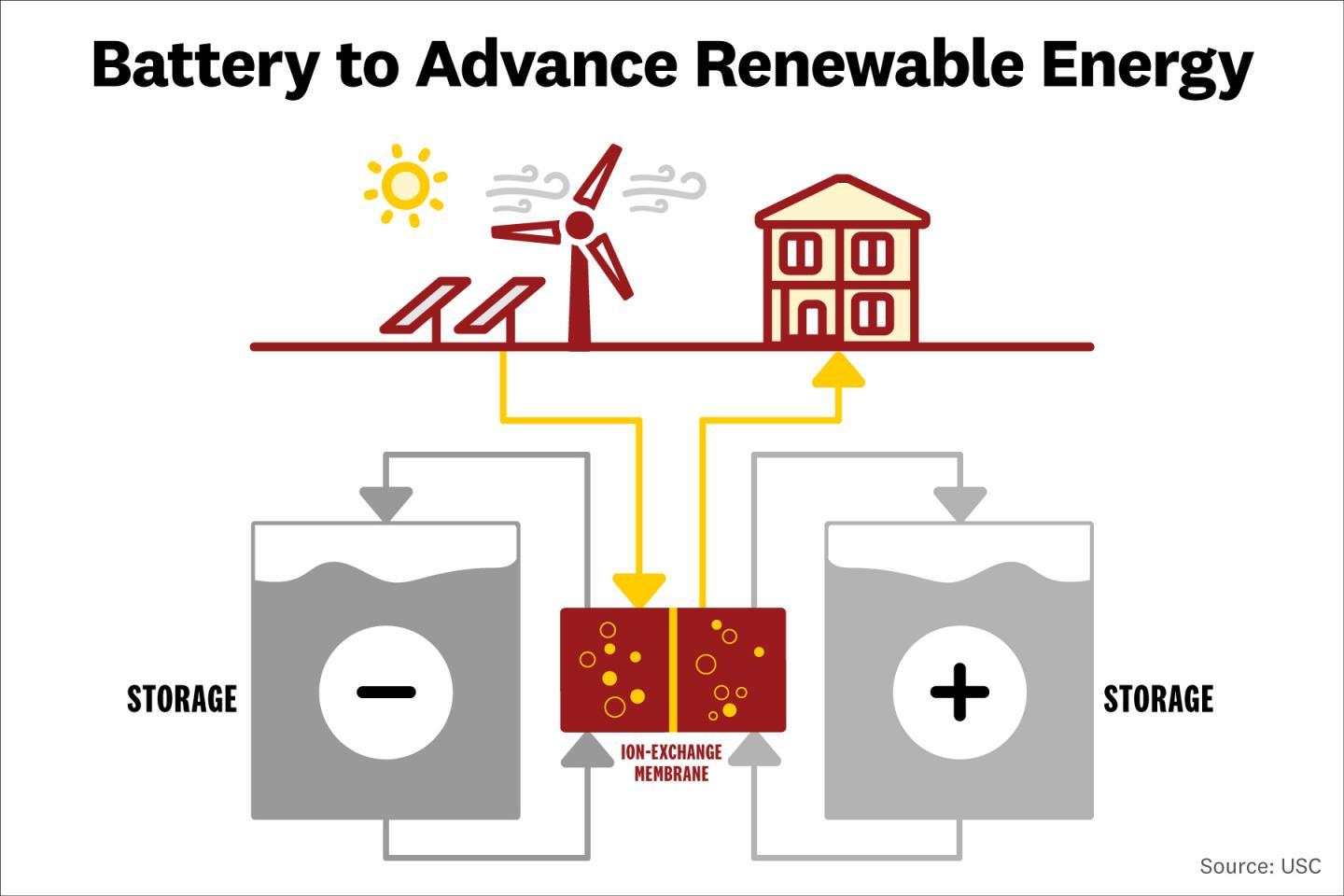
In the so-called redox flow battery, a positive chemical and a negative chemical are stored in separate tanks. The chemicals are pumped in and out of a chamber where they exchange ions across a membrane – flowing one way to charge and the other to discharge.
Though such systems have previously used expensive, dangerous and toxic vanadium and bromine dissolved in acid for their electrolytes in the past, we have seen recent designs that replace those with organic or more environment-friendly alternatives.
USC
For its design, the USC team used a waste product of the mining industry and an organic material that can be made from carbon-based feedstocks, including carbon dioxide, and is already used in other redox flow batteries.
In tests, the iron sulfate solution and Anthraquinone disulfonic acid (AQDS) battery was found able to charge and discharge hundreds of times with “virtually no loss of power.” The researchers say that the inexpensive nature of the materials used could also lead to significant electricity cost savings compared to redox flow batteries using venadium, if manufactured at scale.
“To date there has been no economically viable, eco-friendly solution to energy storage that can last for 25 years,” said lead author on the study Sri Narayan. “Lithium-ion batteries do not have the long-life and vanadium-based batteries uses expensive, relatively toxic materials limiting large-scale use. Our system is the answer to this challenge. We foresee these batteries used in residential, commercial and industrial buildings to capture renewable energy.”
The study has been published in the Journal of The Electrochemical Society.
Source: USC via Eurekalert
Source of Article