Adding rat stem cells to a mouse embryo resulted in a ‘hybrid brain’ in which the rat cells stepped in to restore function when the mouse’s sense of smell was removed, new research has shown. It’s the first time one animal’s cells have been used to rescue another’s senses, and it represents a step forward in regenerative medicine.
What even is a hybrid brain? It sounds like something lifted from the plot of a sci-fi movie – or a whacky ’80s comedy starring Steve Martin – but it’s actually the combination of two species’ cells that develop into an integrated, functional brain. As such, the hybrid brain is important to advancing regenerative neuroscience by creating ‘synthetic’ neural circuits to restore function to damaged or degenerating brains.
In a new study led by researchers at Columbia University’s Irving Medical Center, rat stem cells were introduced to mouse cells very early in their development, producing a mouse brain that used its integrated rat cells to smell.
“We have beautiful models of cells in dishes and 3D cultures called organoids, and they both have their advantages,” said Kristin Baldwin, professor of genetics and development at Columbia University Vagelos College of Physicians and Surgeons and the study’s co-corresponding author. “But none of them allow you to determine if the cells are really functioning at the highest level. This research is starting to show us how we can expand the flexibility of a brain so that it can accommodate other types of inputs, from human-machine interfaces or transplanted stem cells.”
Throesch et al.
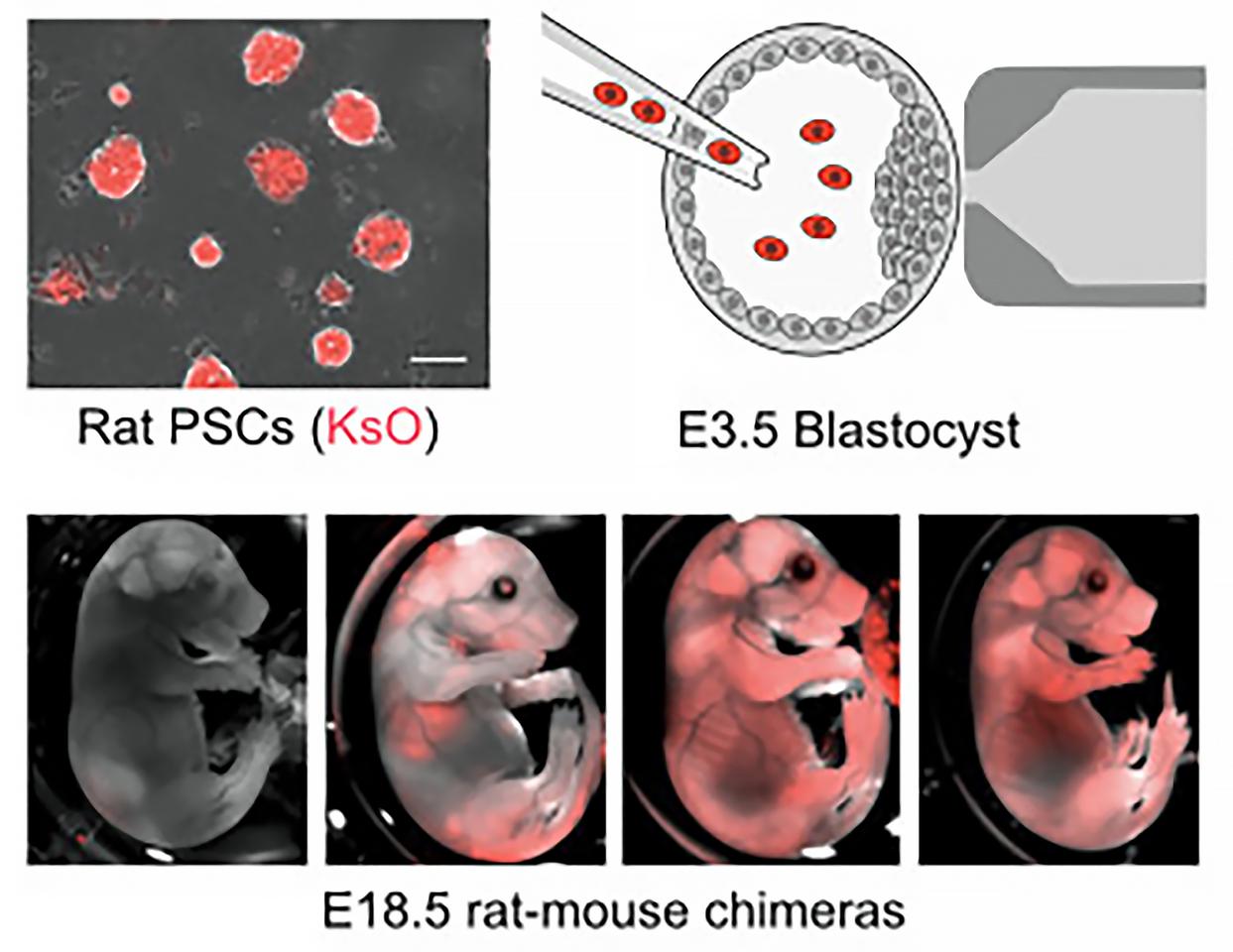
Rat embryonic stem cells were inserted into mouse blastocysts, the cluster of dividing cells made by a fertilized egg, which were then transferred into the uteri of surrogate mouse moms to develop. Despite being evolutionarily different (rat brains develop slower and are larger), the researchers observed that the rat cells grew in sync with the mouse neurons. In the mature rat-mice, or chimeras, the rat cells integrated to form neural circuits throughout the mouse brain and formed active connections with mouse neurons.
“You could see rat cells throughout almost the entire mouse brain, which was fairly surprising to us,” Baldwin said. “It tells us that there are few barriers to insertion, suggesting that many kinds of mouse neurons can be replaced by a similar rat neuron.”
Next was testing the rat cells’ functional abilities and whether they would take over from damaged mouse neurons. The researchers developed mouse models with genetically disabled or ablated – that is, destroyed – olfactory sensory neurons (OSNs), the neurons that detect and transmit information about smells. They found that the rat cells came to the mouse brain’s rescue.
“We hid a cookie in each mouse cage, and we were very surprised to see that they could find it with the rat neurons,” said Baldwin.
However, the mice whose OSNs had been genetically silenced – that is, the neurons were present, just not working – were less successful at finding the cookie than the mice with destroyed OSNs.
“This suggests that adding replacement neurons isn’t plug and play,” Baldwin said. “If you want a functional replacement, you may need to empty out dysfunctional neurons that are just sitting there, which could be the case in some neurodegenerative diseases and also in some neurodevelopmental disorders like autism and schizophrenia.”
One issue the researchers encountered in their study was that the rat cells were randomly distributed in different mice, a barrier to extending the research to other neural systems. They are currently trying to find ways of driving the inserted cells to develop into a particular cell type, which might provide greater precision. Clearing this hurdle would pave the way for creating hybrid brains with primate neurons.
“This would help us get even closer to understanding human disease,” said Baldwin.
The study was published in the journal Cell.
Source: Columbia University Irving Medical Center
Source of Article