Making the successful transition to green energy is dependent on rare earth elements (REEs), which are needed to make essential components in things like hybrid and electric vehicles and wind turbines. Despite their name, these elements aren’t actually rare. The problem is, though, that they’re usually found in low concentrations and are combined, meaning their extraction and separation are expensive, energy- and water-intensive, and generate a lot of waste.
In a new study, researchers from Trinity College at the University of Dublin tested a simple process of REE recovery that relies on a cheap, sustainable resource: the humble eggshell.
“This study presents a potential innovative use of waste material that not only offers a sustainable solution to the problem of rare earth element recovery but also aligns with the principles of circular economy and waste valorization,” said Remi Rateau, from the College’s School of Natural Sciences and the study’s lead and corresponding author.
Trinity College Dublin/University of Dublin
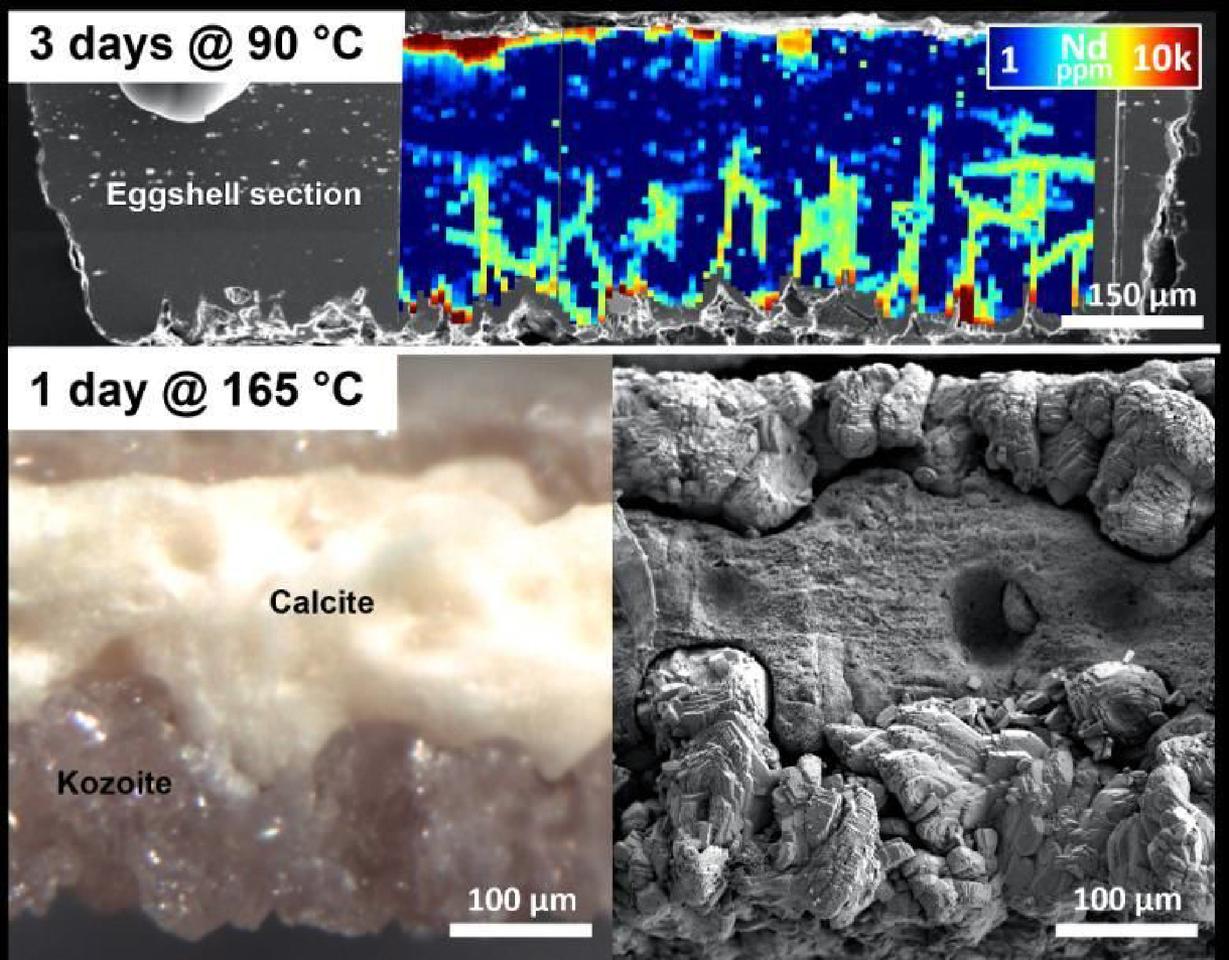
The researchers placed waste hen eggshells in solutions containing REEs and heated them to temperatures ranging from 25 °C (77 °F) up to 205 °C (401 °F) for up to three months. They observed that the REEs diffused – that is, they moved from a region of high concentration to one of low concentration – into the eggshells along the calcium carbonate (calcite) boundaries and the organic matrix, a complex mixture of organic material, including protein, that’s intimately associated with the outer calcite shell.
The REEs formed new minerals at higher temperatures on the eggshell’s surface. At 90 °C (194 °F), kozoite formed on the surface of the dissolving calcite. At 165 °C (329 °F) and 205 °C, the researchers noted that the calcite dissolved completely and was replaced by polycrystalline kozoite. At 205 °C, the kozoite was slowly replaced by bastnäsite, the stable rare earth mineral typically used to extract REEs for technological applications.
The researchers say their simple, low-cost and environmentally friendly process could help meet the increasing demand for REEs as the world embraces more green energy technologies.
“By transforming eggshell waste into a valuable resource for rare earth recovery, we address critical environmental concerns associated with traditional extraction methods and contribute to the development of greener technologies,” said Juan Diego Rodriguez-Blanco, lead researcher on the study.
The study was published in the journal ACS Omega.
Source: Trinity College Dublin
Source of Article