South Korean researchers have shown a stem-cell-based topical solution can regrow hair in both male and female subjects with common pattern baldness. The small, randomized, placebo-controlled trial found the treatment both safe and effective, with larger trials hoped to validate the results in more diverse populations.
The most common form of age-related baldness is known as androgenetic alopecia (AGA). Around half of men and women over the age of 50 will deal with some form of this hair loss, from a receding hairline to more general hair thinning.
The new trial focused on a type of stem cell abundantly found in adult fat tissue, called adipose tissue‐derived stem cells (ADSCs), which have been found to secrete a number of growth hormones vital to hair development. Smaller studies have demonstrated ADSCs can regrow hair in male and female subjects but this is the first robust human clinical trial to test a novel topical treatment against a placebo.
“Recent studies have shown that ADSCs promote hair growth in both men and women with alopecia,” explains Sang Yeoup Lee, from Pusan National University in South Korea. “However, no randomized, placebo-controlled trial in humans has explored the effects and safety of adipose-derived stem cell constituent extract (ADSC-CE) in AGA. We aimed to assess the efficacy and tolerability of ADSC-CE in middle-aged patients with AGA in our study, hypothesizing that it is an effective and safe treatment agent.”
This small, double-blinded trial gathered 38 subjects, nine of whom were women. Half received the ADSC-CE topical solution and half received a placebo, with directions to apply the solution to the scalp twice a day for 16 weeks.
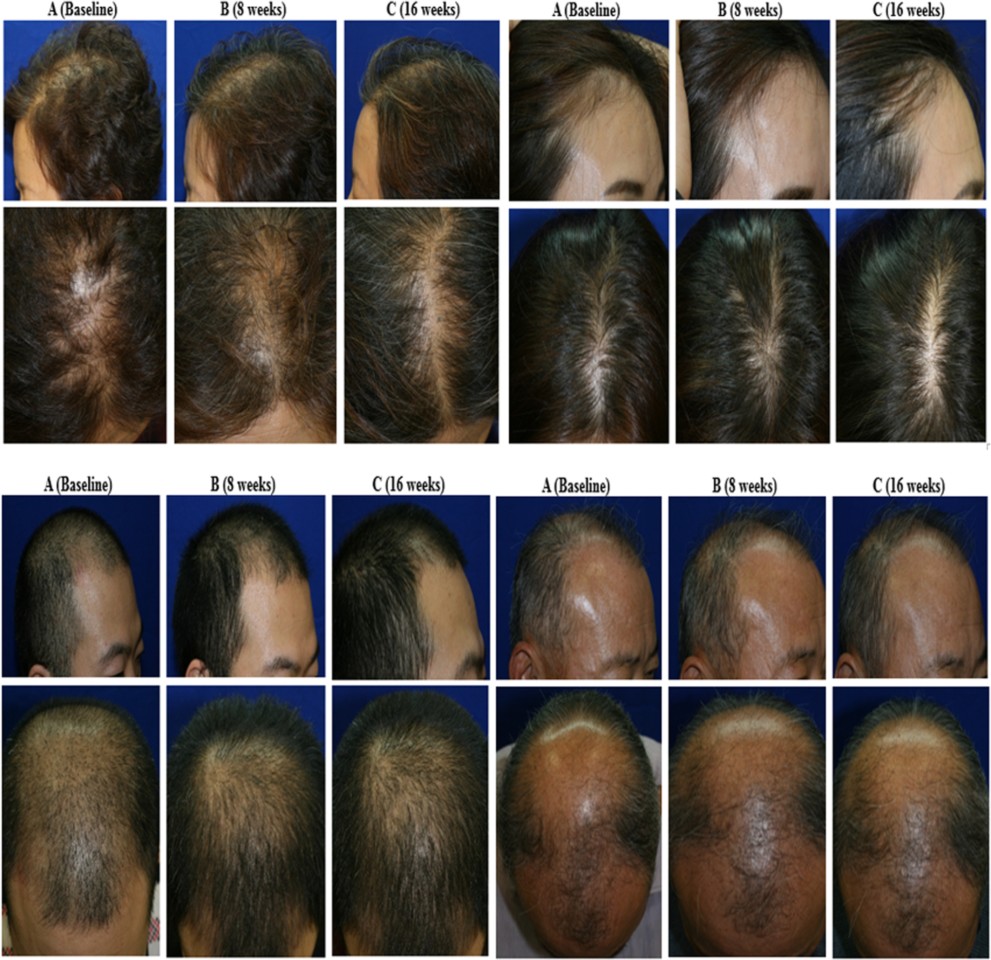
Tak/Lee/Cho/Kim – STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals, Inc. on behalf of AlphaMed Press
After 16 weeks the researchers found mean hair density in the intervention group had increased by 28.1 percent, compared to 7.1 percent in the placebo group. Mean hair thickness increased 14.2 percent for those using the active solution, compared to 6.3 percent in the control.
The results mirror prior studies exploring the efficacy of ADSCs in stimulating hair growth, however, many other trials worked with different delivery methods, often using injectable techniques. This research suggests the novel ADSC-CE topical solution developed by the researchers is likely as effective as more invasive therapies. And, of course, this simple topical solution is a much easier treatment method than an injectable preparation potentially requiring frequent visits to a clinical environment.
“Our findings suggest that the application of the ADSC-CE topical solution has enormous potential as an alternative therapeutic strategy for hair regrowth in patients with AGA, by increasing both hair density and thickness while maintaining adequate treatment safety,” concludes Lee. “The next step should be to conduct similar studies with large and diverse populations in order to confirm the beneficial effects of ADSC-CE on hair growth and elucidate the mechanisms responsible for the action of ADSC-CE in humans.”
The new study was published in the journal Stem Cells Translational Medicine.
Source: AlphaMed Press
Source of Article